38 structure function claims on dietary supplement labels
› food › dietary-supplements-guidanceDietary Supplement Labeling Guide: Chapter VI. Claims | FDA What health claims can be used on dietary supplement labels? ... What must I do when making structure/function claims in my products' labeling? You must (1) have substantiation that such statement ... Structure/Function Claims - nutritionlabs.us Dietary Supplements. Structure/function claims have historically appeared on the labels of conventional foods and dietary supplements as well as drugs. The Dietary Supplement Health and Education Act of 1994 (DSHEA) established some special regulatory requirements and procedures for structure/function claims and two related types of dietary ...
Stock Market The "US Dietary Supplements - Regulatory Compliance Requirements, Product Claims, Labeling Issues and FDA Updates Course" training has been added to ResearchAndMarkets.com's offering.. This training will review the dietary supplement regulations in the USA and explain how to verify that your products are compliant with the most recent regulations and provisions.

Structure function claims on dietary supplement labels
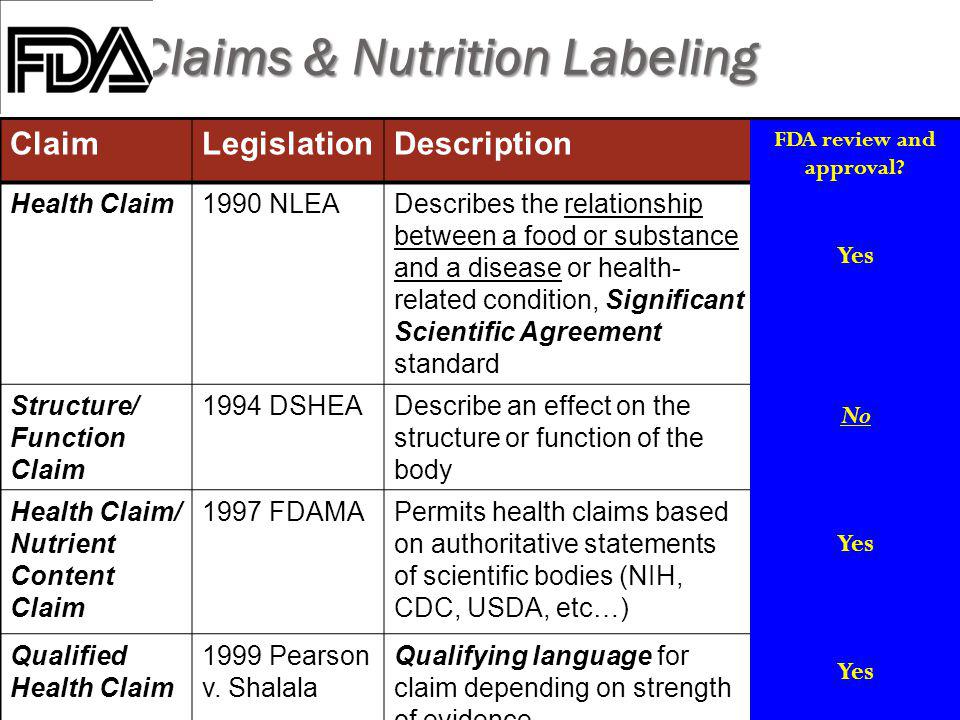
› food › information-consumers-usingQuestions and Answers on Dietary Supplements | FDA May 06, 2022 · These dietary supplement claims are subject to the same requirements as structure/function claims, including the disclaimer that must accompany the claim and the requirement for the manufacturer ... Understanding Dietary Supplement Claims | Consumer Healthcare ... - CHPA 1. Structure/function claims are the most common, permissible claim used for dietary supplements. DSHEA established special requirements for structure/function claims, including claims that are related to general well-being and nutrient deficiencies. Structure function claims can be easily confused with disease claims, which refer to a specific ... PDF Structure Function Claims: Substantiation Pitfalls A Structure Function Claim A statement describing the effect of a nutrient or dietary ingredient on a structure or function of the human body or characterizing the documented mechanism of such effect. These may appear on the labels of foods, dietary supplements, or drugs. • The definition of what a viable structure function claim varies though
Structure function claims on dietary supplement labels. Structure/Function Claim Notification for Dietary Supplements The law states that a dietary supplement may bear certain statements on its label or in its labeling if the claim (s) meets certain requirements, if the entities making the claims have... › regulatory-information › search-fdaGuidance on Substantiation for Dietary Supplement Claims Please see the Federal Register of January 6, 2000 (65 FR 1000, codified at 21 CFR 101.93) for the final rule defining structure/function claims for dietary supplements and the January 9, 2002 ... › structurefunction-claimsStructure/Function Claims | FDA Mar 07, 2022 · Structure/Function Claims for dietary supplements ... appeared on the labels of conventional foods and dietary supplements as well as drugs. The Dietary Supplement Health and Education Act of 1994 ... › food › food-labeling-nutritionLabel Claims for Conventional Foods and Dietary Supplements Mar 07, 2022 · The Dietary Supplement Health and Education Act of 1994 (DSHEA) established some special regulatory requirements and procedures for using structure/function claims and two related types of dietary ...
How To Make Structure/Function Claims That Comply With DSHEA The Dietary Supplement Health and Education Act of 1994 (DSHEA) established some special regulatory requirements and procedures for structure/function claims and two related types of dietary supplement labeling claims, claims of general well-being and claims related to a nutrient deficiency disease. › regulatory-information › search-fdaSmall Entity Compliance Guide on Structure/Function Claims What are structure/function claims? The Dietary Supplement Health and Education Act of 1994 (DSHEA) added section 403(r)(6) ... Dietary supplement labels or labeling may, subject to the ... Dietary Supplements Claims, Labels and Regulations | NSF Structure/function claims refer to the supplement's effect on the body's structure or function, including its overall effect on a person's well-being. Examples of structure/function claims include "Calcium builds strong bones" and "Antioxidants maintain cell integrity". Nutrient Content Claims Should function claims be allowed on dietary supplements? Which claims can or Cannot be made on dietary supplement labels? Basically, dietary supplements cannot make 'disease' claims (for example: 'this supplement shrinks tumors'). Dietary supplements that make disease claims will be regulated by the FDA as drugs. Dietary supplements can make 'structure/function' claims (for example, 'calcium builds ...
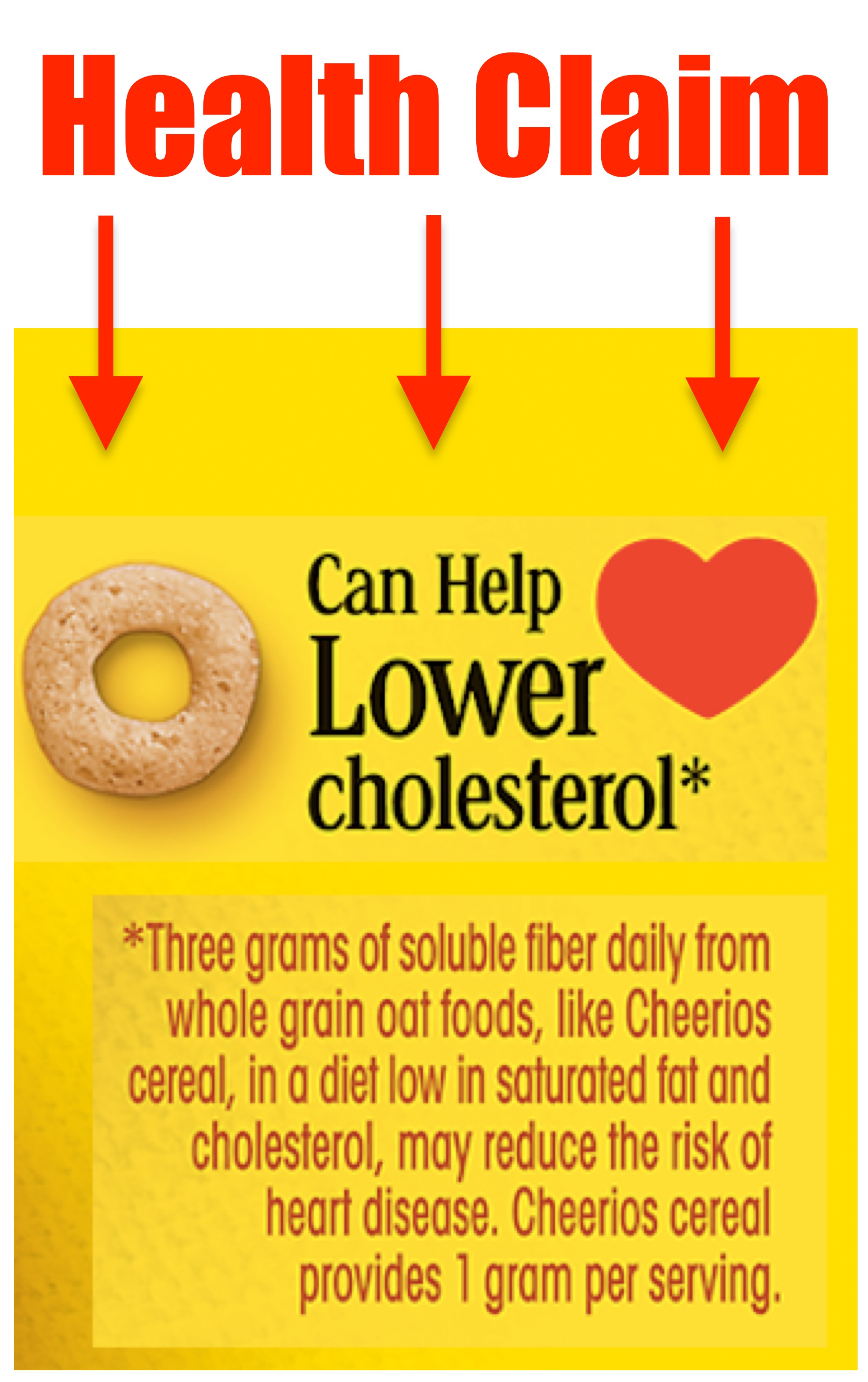
Structure-function Claims On Dietary Supplements Labels Exipure, which contains amun and cork bark, is a natural weight loss supplement. Amun cork bark contains an amino acid called amygdalin. Exipure raises brown sugar levels, which is a vitally important dietary factor for burning stubborn belly fat. Drs. James Wilkins and Lam developed Exipure and it is vegetarian-friendly. en.wikipedia.org › wiki › Vitamin_EVitamin E - Wikipedia The U.S. Food and Drug Administration initiated a process of reviewing and approving food and dietary supplement health claims in 1993. Reviews of petitions results in proposed claims being rejected or approved. If approved, specific wording is allowed on package labels. In 1999, a second process for claims review was created. PDF Permissible vs. Impermissible Structure/Function Claims for Dietary ... Structure/Function Claims for Dietary Supplements. 2 THE BASICS: ... the heart symbol on product label and labeling is an impermissible heart disease prevention claim.) 14 ... No more than thirty (30) days after a supplement bearing a structure/function claim is marketed, the manufacturer, packer, or distributor of the FDA Labeling Guidance: Making Structure/Function Claims for Dietary ... the fda permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure or function in humans" or characterize the "documented mechanism" by which the nutrient acts to maintain such structure or function, but do not claim to "diagnose, …
PDF Structure Function Claims: Substantiation Pitfalls A Structure Function Claim A statement describing the effect of a nutrient or dietary ingredient on a structure or function of the human body or characterizing the documented mechanism of such effect. These may appear on the labels of foods, dietary supplements, or drugs. • The definition of what a viable structure function claim varies though
Understanding Dietary Supplement Claims | Consumer Healthcare ... - CHPA 1. Structure/function claims are the most common, permissible claim used for dietary supplements. DSHEA established special requirements for structure/function claims, including claims that are related to general well-being and nutrient deficiencies. Structure function claims can be easily confused with disease claims, which refer to a specific ...
› food › information-consumers-usingQuestions and Answers on Dietary Supplements | FDA May 06, 2022 · These dietary supplement claims are subject to the same requirements as structure/function claims, including the disclaimer that must accompany the claim and the requirement for the manufacturer ...
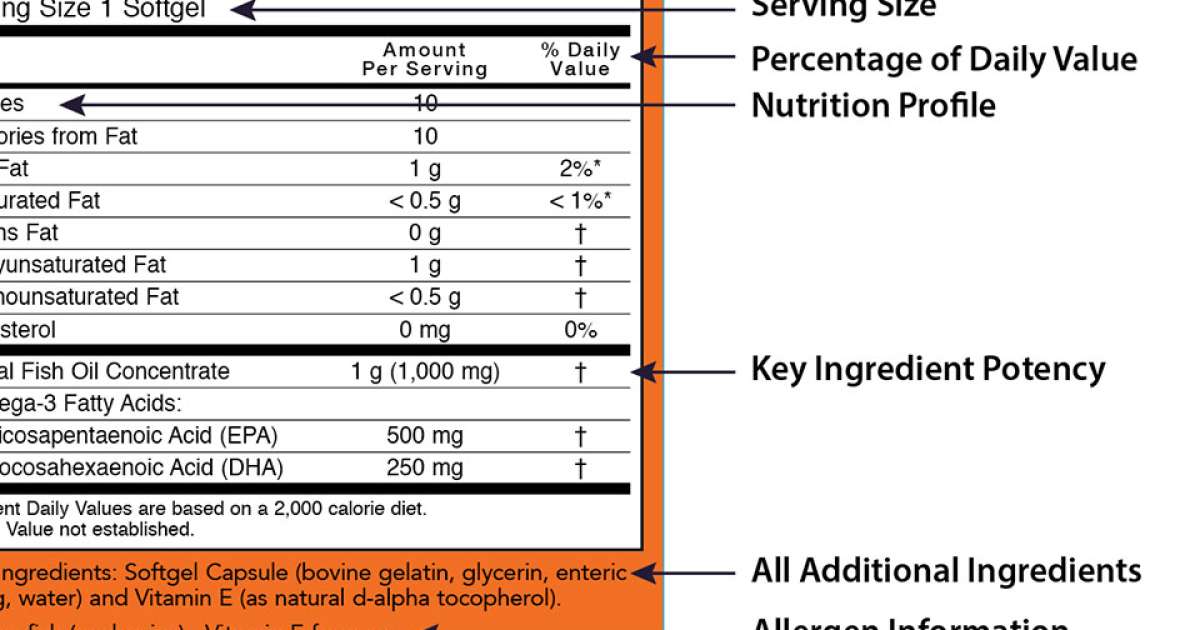























:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/3650624/quakerlabel-shelf.0.jpg)

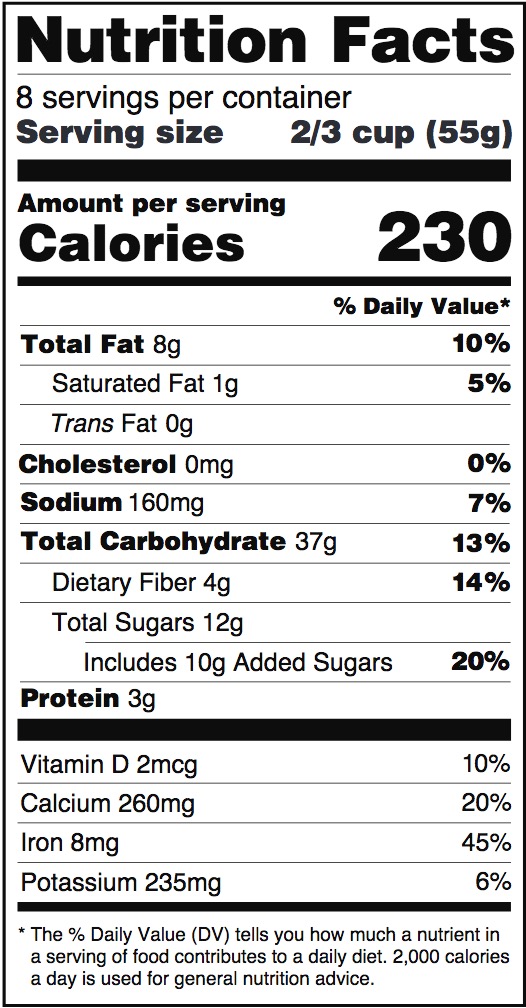


Post a Comment for "38 structure function claims on dietary supplement labels"