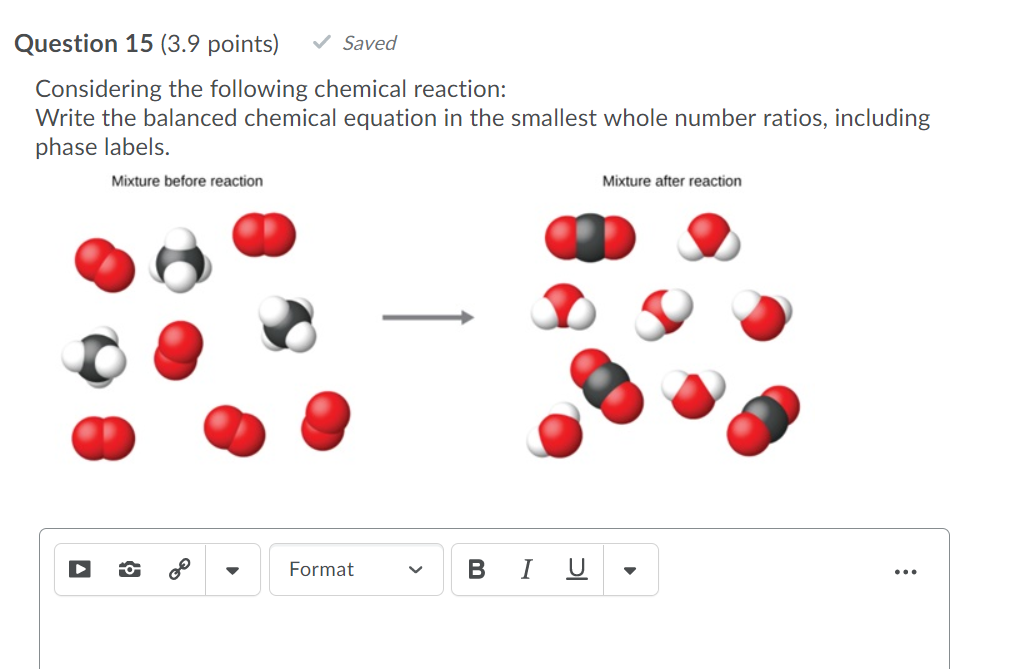
39 phase labels in chemical equations
What are Chemical Equations? Detailed Explanation, Examples - BYJUS An example of an ionic chemical equation is provided below. Chemical Equation: CaCl 2 + 2AgNO 3 → Ca (NO 3) 2 + 2AgCl↓. Ionic Equation: Ca 2+ + 2Cl - + 2Ag + + 2NO 3- → Ca 2+ + 2NO 3- + 2AgCl↓. Comparing the reactants and the products of the ionic equation and the chemical equation, it can be observed that the Ca 2+ ( calcium ion ... Definition of dissolve and the state label (aq) in chemical equations A colloid is a heterogeneous phase. But above all, do not worry too much about these minor symbolism issues. A chemical equation is not meant to convey everything. It is just a shorthand notation to describe chemical reactions. A real experiment or a research paper should describe the experimental condition with sufficient details in such a way ...
Phase line calculator differential equations The geometry of this foliation depends on the nature .... 2009. 9. 4. · Phase Line Diagram A phase line diagram for the autonomous equation y0= f(y) is a line segment with labels sink, source or node, one for each root of f(y) = 0, i.e., each equilibrium; see Figure1. It summarizes the contents of a direction field and threaded curves ...

Phase labels in chemical equations
Many chemical equations also include phase labels for Many chemical equations also include phase labels for the substances s for solid from AGRICULTUR 847,272 at Eastern Visayas State University - Tacloban City Main Campus 3 Steps for Balancing Chemical Equations - ThoughtCo To do this, you need to be familiar with the properties of various compounds or you need to be told what the phases are for the chemicals in the reaction. Oxides are solids, hydrogen forms a diatomic gas, tin is a solid, and the term ' water vapor ' indicates that water is in the gas phase: SnO 2 (s) + 2 H 2 (g) → Sn (s) + 2 H 2 O (g) End-of-Chapter Material - Introductory Chemistry- 1st Canadian Edition Write a chemical reaction for the boiling of water, including the proper phase labels. Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why. 4 Na(s) + 2 Cl 2 (g) → 4 NaCl(s)
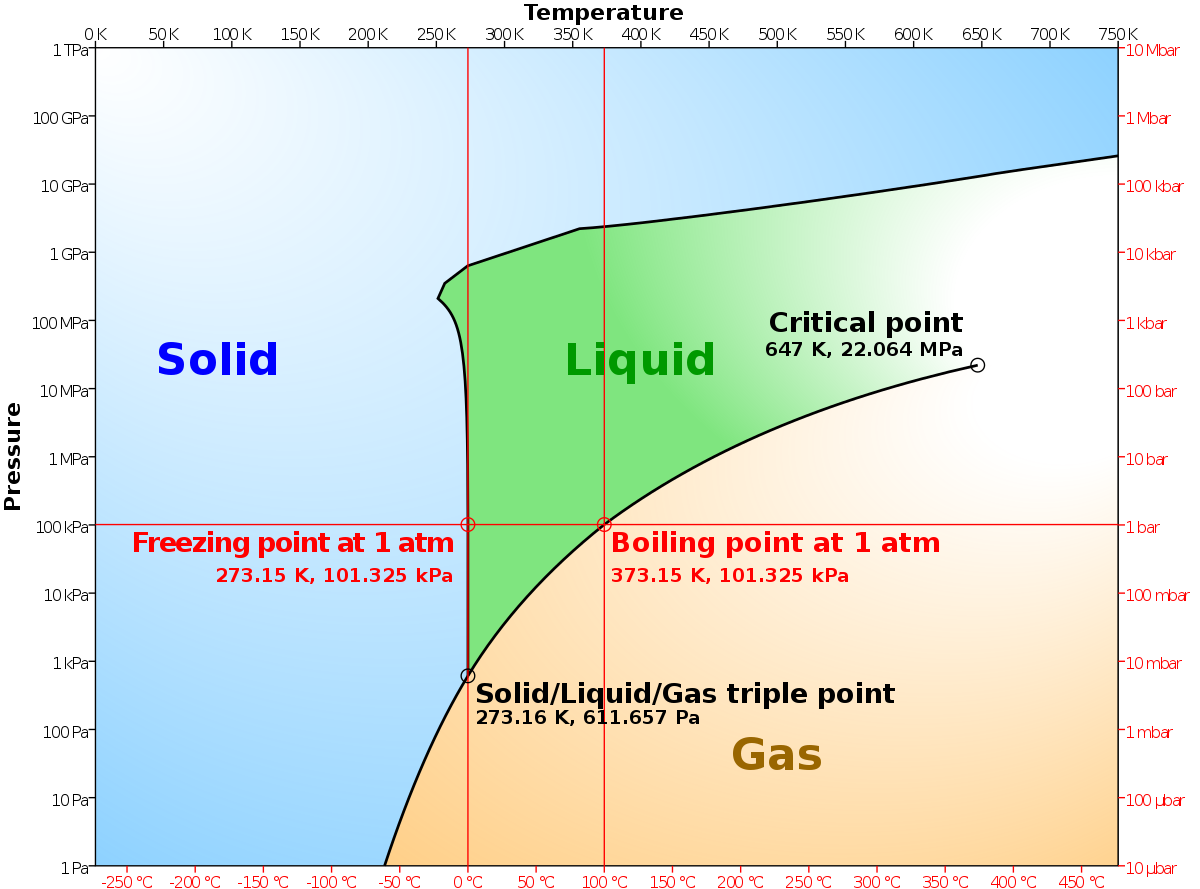
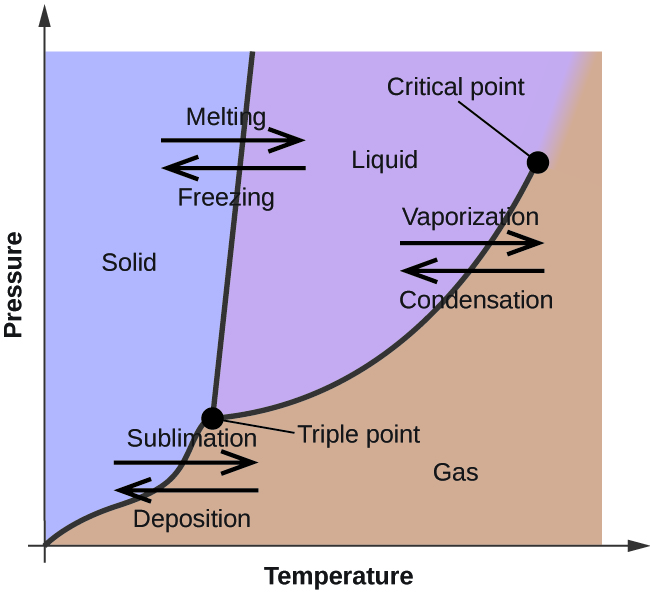
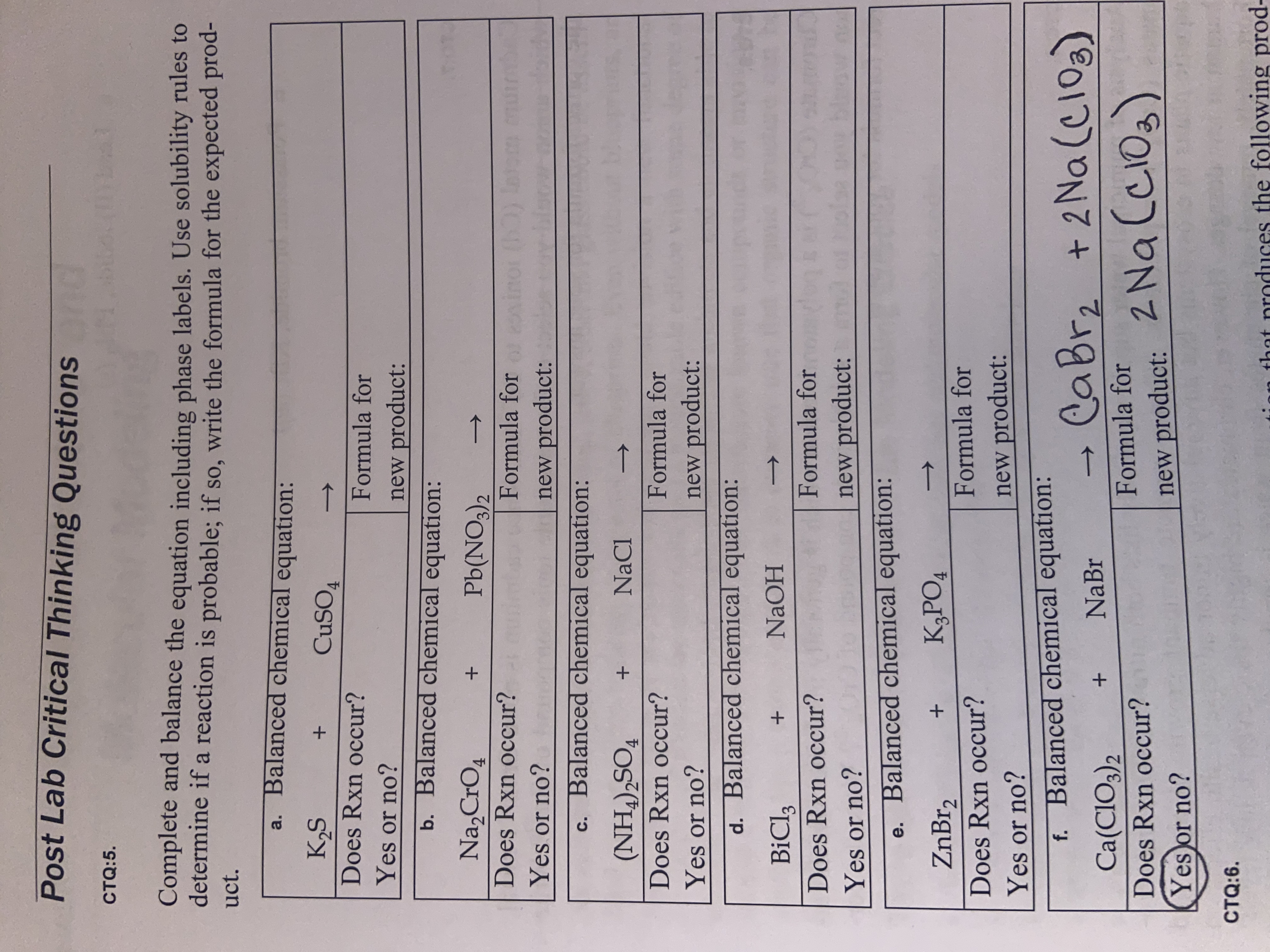
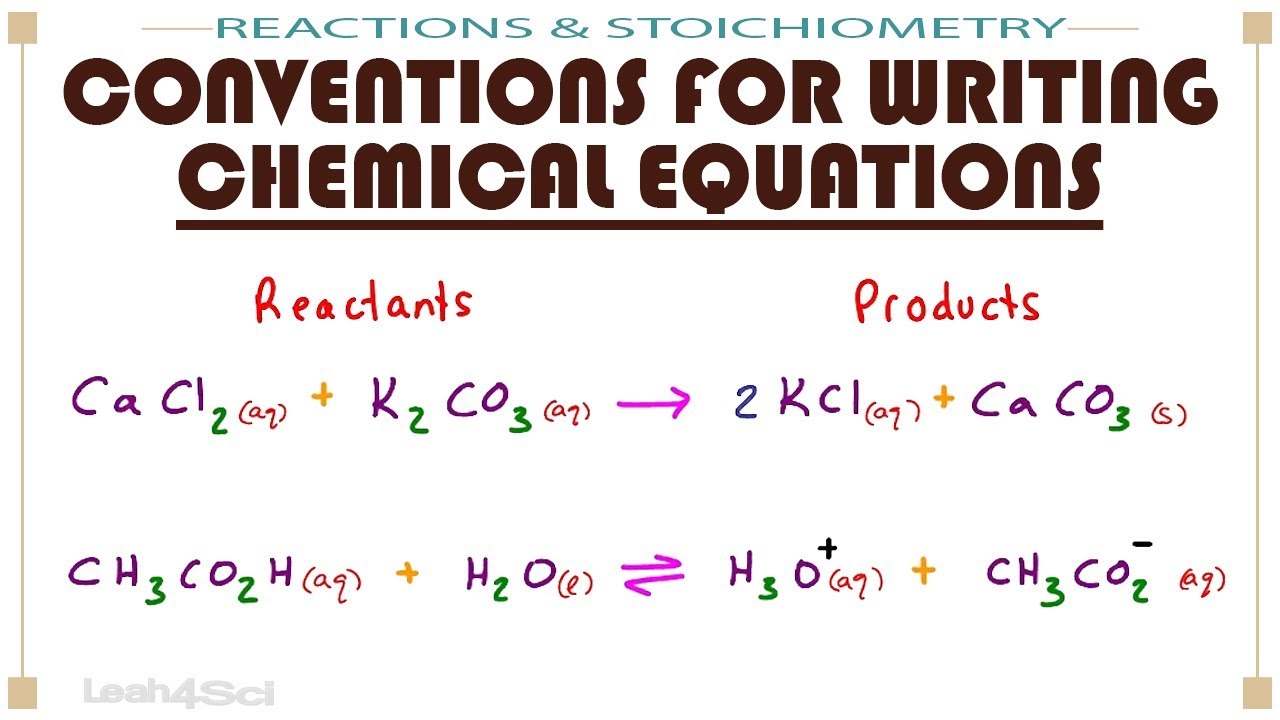
Phase labels in chemical equations. UpToDate {{configCtrl2.info.metaDescription}} Sign up today to receive the latest news and updates from UpToDate. Sign Up EXAM #2: Chapter 4 Flashcards | Quizlet Write a chemical reaction for the boiling of water, including the proper phase labels. ... 2. Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. ... 3. Explain why 4Na (s)+2ClX2 (g) 4NaCl (s) should not be considered a proper chemical equation. Correlative operando microscopy of oxygen evolution ... - Nature May 05, 2021 · An observed mass change corresponding to 0.56 ± 0.2 moles of OH − inserted per mole of Co suggests an intermediate phase of CoO 2 H 1.5 ·0.5H 2 O according to the reaction shown below; we ... State symbols and phase changes | StudyPug The phase can affect how reactive a substance is, but changing phase (a physical change) is not the same as changing the substance (a chemical change). A fully detailed chemical equation will show the state (or phase) of matter that the atoms or molecules are in. These states are: Solid, given the symbol (s) Liquid, given the symbol (l)
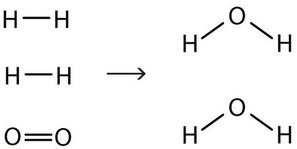
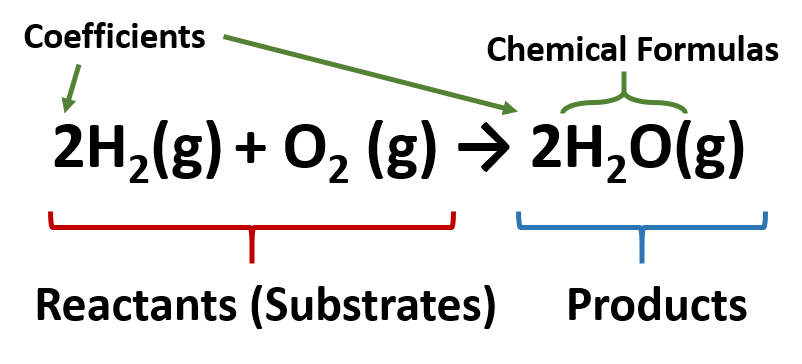
The Chemical Equation | Introductory Chemistry - Lumen Learning Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, 2 NaHCO3(s) →200°C Na2CO3(s) + CO2(g) + H2O (ℓ) Key Takeaways Phase Labels - YouTube Clark College Tutoring and Writing Center tutors Kevin Martin and Joey Smokey explain the usage of phase labels in chemical reactions such as (s), (l), (g), ... Molecular orbital - Wikipedia Sigma and pi labels for MOs. The type of interaction between atomic orbitals can be further categorized by the molecular-orbital symmetry labels σ (sigma), π (pi), δ (delta), φ (phi), γ (gamma) etc. These are the Greek letters corresponding to the atomic orbitals s, p, d, f and g respectively. The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Write and balance the chemical equation that represents nitrogen and hydrogen reacting to produce ammonia, NH 3. Answer N 2 + 3H 2 → 2NH 3 Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water).
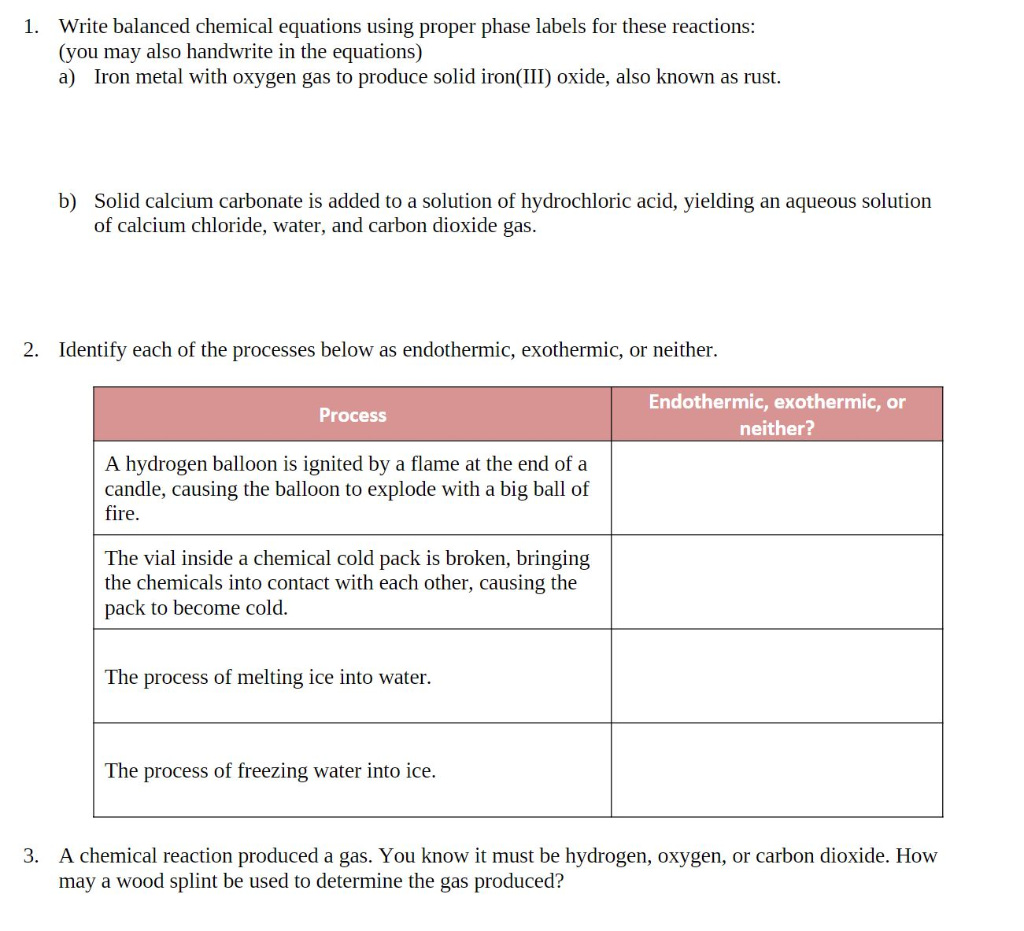
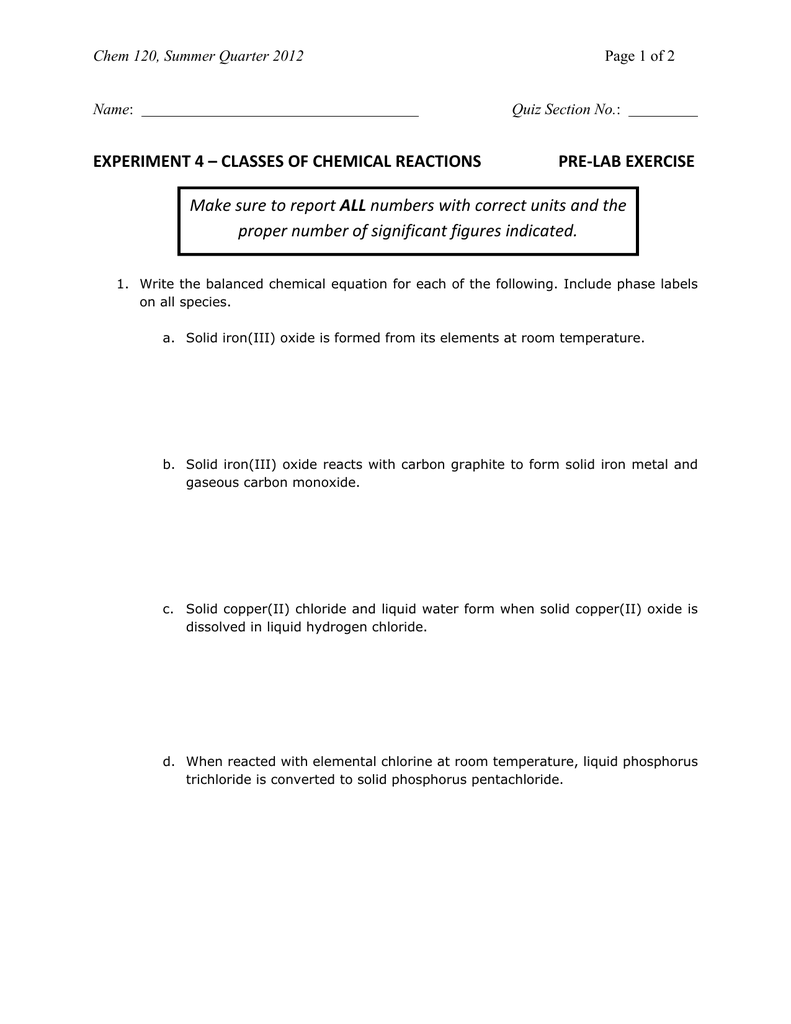
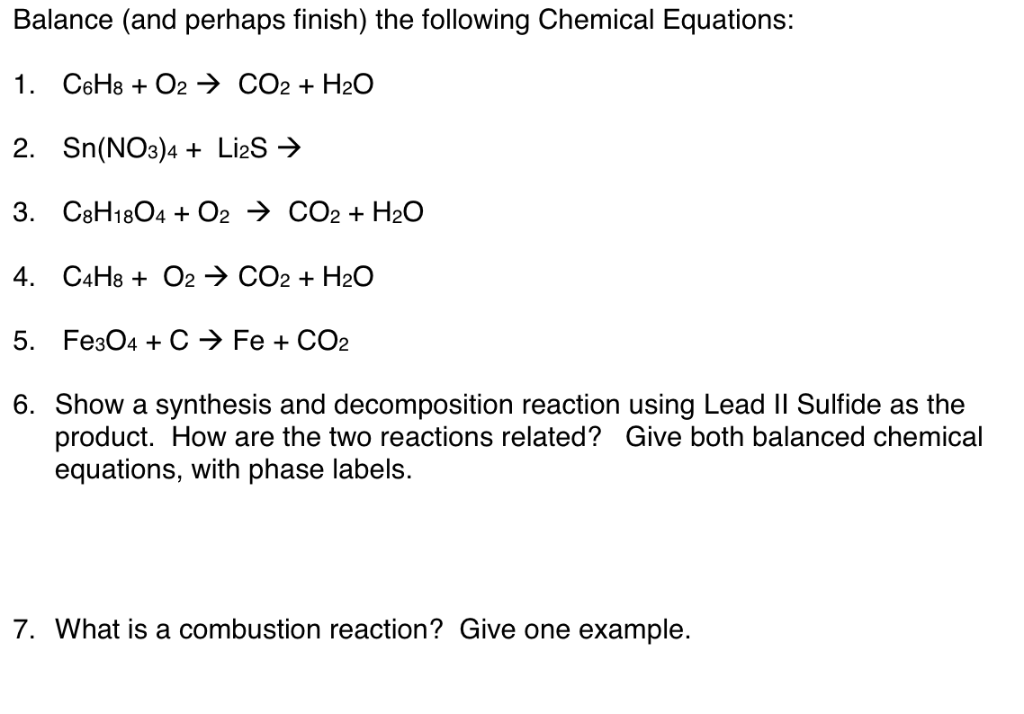
Solved 1. Write balanced chemical equations using proper | Chegg.com 1. Write balanced chemical equations using proper phase labels for these reactions: (you may also handwrite in the equations) a) Iron metal with oxygen gas to produce solid iron (III) oxide, also known as rust. Home Page: Annals of Emergency Medicine ACEP Member Login. ACEP Members, full access to the journal is a member benefit. Use your society credentials to access all journal content and features. SOLVED:16. Write a balanced equation (including phase labels) for the ... we are just looking to balance some chemical equations. So the first one we have a gcl in the solid state. I won't list. I went right down the states but I will say them aloud just to save a bit of times what a gcl solid at two and H. Three A quits. That gives us A G. And H. 32 plus acquis Art C. L minus liquids. The next example we've got C R E N two cl two cl acquits R two H 20. pycse - Python3 Computations in Science and Engineering Note that only one root is real (and even then, we have to interpret 0.j as not being imaginary. Also, in a cubic polynomial, there can only be two imaginary roots). In this case that means there is only one phase present.
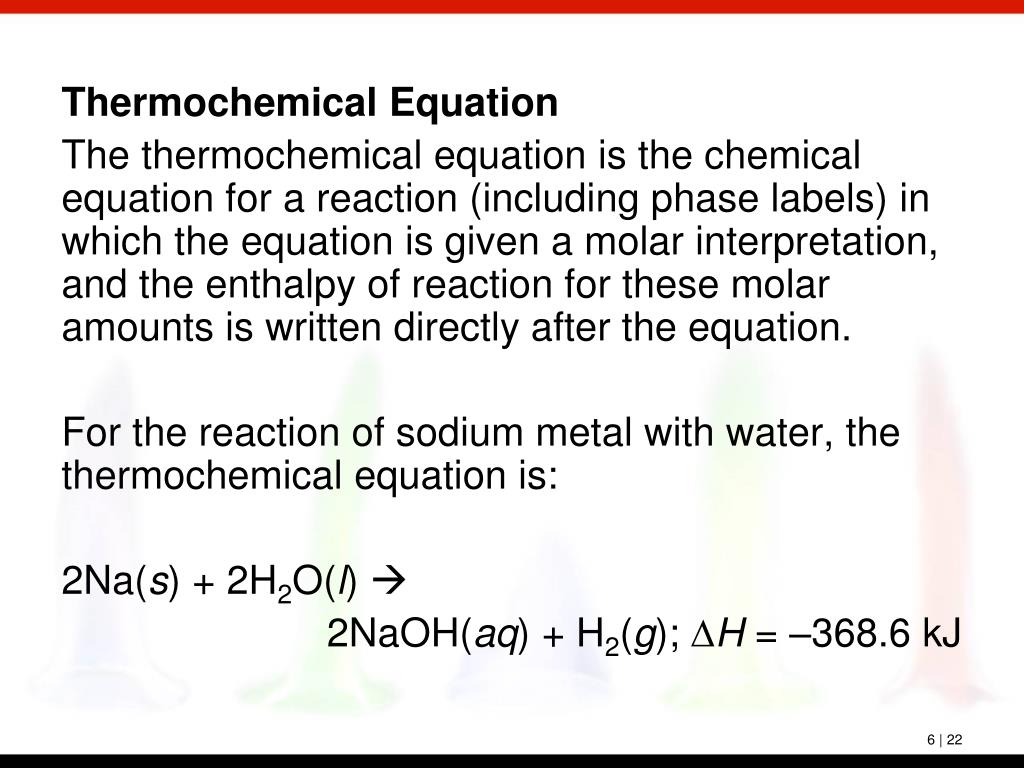
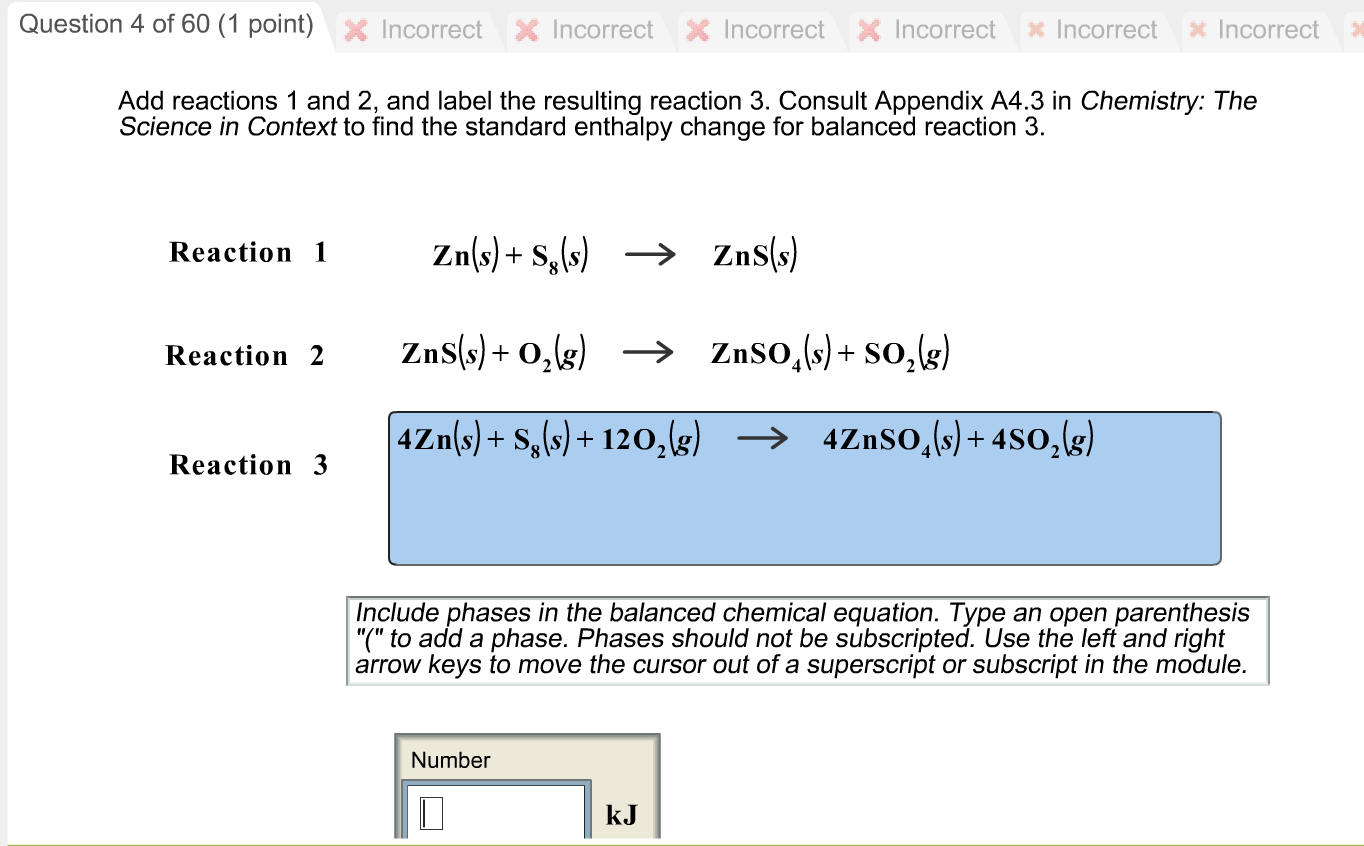
Solved 1. Write a balanced thermochemical equation with | Chegg.com Question: 1. Write a balanced thermochemical equation with phase labels for the Haber process with the heat energy as part of the equation. (3 pts) 2. What is the theoretical yield of ammonia (in grams) if 16.55 grams of nitrogen gas and 10.15 grams of hydrogen gas are allowed to react? (9 pts) 3.
Chapter 5 - Chemical Reactions and Equations - CHE 105/110 ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). ... phase label. To balance this equation, we need two phosphate ions and three calcium ions; we end up with six water molecules to balance the equation: 2H 3 PO 4 (aq) + 3Ca(OH) 2 ...
End-of-Chapter Material | Introductory Chemistry - Lumen Learning Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation.
End-of-Chapter Material - Introductory Chemistry - 1st Canadian Edition Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl 2 (g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H 2 (g) + ½O 2 (g) → H 2 O (ℓ) should not be considered a proper chemical equation.
End-of-Chapter Material Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl2(g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2O2(g) → H2O (ℓ) should not be considered a proper chemical equation.
4.E: Chemical Reactions and Equations (Exercises) Jun 23, 2022 · Chemical equations can also be used to represent physical processes. Write a chemical reaction for the boiling of water, including the proper phase labels. Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why. 4Na(s) + 2Cl 2 (g ...
Chemical Reactions and Equations - GitHub Pages Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). ... According to the solubility rules, Ca 3 (PO 4) 2 is insoluble, so it has an (s) phase label. To balance this equation, we need two phosphate ions and three calcium ions; we end ...
End-of-Chapter Material - Introductory Chemistry- 1st Canadian Edition Write a chemical reaction for the boiling of water, including the proper phase labels. Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why. 4 Na(s) + 2 Cl 2 (g) → 4 NaCl(s)
3 Steps for Balancing Chemical Equations - ThoughtCo To do this, you need to be familiar with the properties of various compounds or you need to be told what the phases are for the chemicals in the reaction. Oxides are solids, hydrogen forms a diatomic gas, tin is a solid, and the term ' water vapor ' indicates that water is in the gas phase: SnO 2 (s) + 2 H 2 (g) → Sn (s) + 2 H 2 O (g)
Many chemical equations also include phase labels for Many chemical equations also include phase labels for the substances s for solid from AGRICULTUR 847,272 at Eastern Visayas State University - Tacloban City Main Campus

















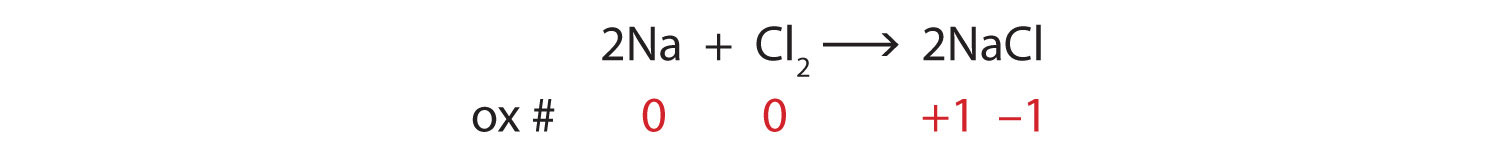


Post a Comment for "39 phase labels in chemical equations"